nickel valence electrons|valence electrons chart : Cebu Electron configuration for nickel. The history of Nickel. Periodic table history. Identifiers. List of unique identifiers for Nickel in various chemical registry databases. Nickel is a .
Play & Win Best Online Sakla Live in Philippines . Get Slots & Poker Games for Free , New Members Registration to Enjoy . Join Now !
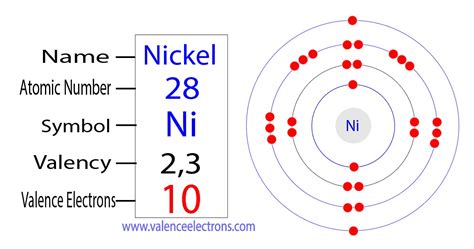
nickel valence electrons,Mar 23, 2023 It has the variable valency since Nickel is a transition chemical element. Nickel has a fixed valency of +2 in its pure oxidation state. The valency of this chemical . Valences of the Elements Chemistry Table. You may assume that the . To find the number of valence electrons for Nickel (Ni) we need to look at its electron configuration. This is necessary because Ni is a transition metal (d block .Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row .Electron configuration for nickel. The history of Nickel. Periodic table history. Identifiers. List of unique identifiers for Nickel in various chemical registry databases. Nickel is a . Valence electrons are the outer-shell electrons of an atom. Valence electrons determine the reactivity of an atom. Atoms have a tendency to have eight . The nickel consists of 28 electrons in the atomic shell in 4 orbits. Electron configuration is the distribution of electrons in the orbits of atoms or molecules. The electronic configuration of nickel is: 1s 2 2s 2 .nickel valence electronsValence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called . Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .In this case, the nickel atom carries a positive charge. Ni – 2e – → Ni 2+. Here, the electron configuration of nickel ion (Ni 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 8. This nickel ion (Ni 2+) has twenty-eight protons, thirty-one .
Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd .
Comprehensive information for the element Nickel - Ni is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions. . Valence Electrons: 3d 8 4s 2. Chemical Properties of Nickel. Electrochemical Equivalent: 1.095g/amp-hr; Electron Work .
Nickel at Ground State: Ni: 8 d-electrons = [Ar] 4s 2 3d 8. Nickel with an Oxidation State of +2: Ni 2 +: [Ar] 4s 0 3d 8. Or simply Ni 2 +: [Ar] 3d 8. In this example, the electron configuration for Ni 2 + still kept its 3d 8, but lost the 4s 2 (became 4s 0) because the s-orbital has the highest
2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) If you're given the configuration from the get-go, you can skip to the next step.Counting electrons on the ligands and the metal. There is a wrinkle in this process if charges are involved. For example, if the free ligands are not neutral, but charged, you need to adjust the electron count on the metal. The metal may be an ion, not an atom, so the electron count will be lower. If donor atoms have formal charges, adjust the .

Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..

The stronger pull (higher effective nuclear charge) experienced by electrons on the right side of the periodic table draws them closer to the nucleus, making the covalent radii smaller. Figure 8.4.2 8.4. 2: Within each period, the trend in atomic radius decreases as Z increases; for example, from K to Kr.valence electrons chart The stronger pull (higher effective nuclear charge) experienced by electrons on the right side of the periodic table draws them closer to the nucleus, making the covalent radii smaller. Figure 8.4.2 8.4. 2: Within each period, the trend in atomic radius decreases as Z increases; for example, from K to Kr.
Nickel has eight electrons in the 3d orbital and two electrons in the 4s orbital, which means nickel has 10 total valence electrons. The reason it has 10 is because nickel is a transition metal, so the d and s electrons can participate in chemical bonding. Valence electrons are the electrons of an atom that can participate in . Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called inner-shell electrons and are not involved directly in the element's reactivity, or in the formation of compounds. Lithium has a single electron in the second . Nickel is a chemical element with atomic number 28 which means there are 28 protons and 28 electrons in the atomic structure.The chemical symbol for Nickel is Ni. Electron Configuration and Oxidation States of Nickel. Electron configuration of Nickel is [Ar] 3d8 4s2. Possible oxidation states are +2,3. Electron Configuration The Valence Electrons Calculator is a powerful tool used in chemistry to determine the number of valence electrons in an atom based on its position in the periodic table. Valence electrons are crucial for understanding an element’s chemical properties and its ability to form bonds with other elements. See also H₂O Molar Mass Calculator . By definition, the highest principle quantum number is the valence shell. In the case of Nickel, n = 4 is the highest QN and contains 2 electrons (max allowed) in the s-orbital. Answer link. 2 Ni: [Ar]3d^"8"4s^2 By definition, the highest principle quantum number is the valence shell. As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into the same group on Mendeleev's periodic table. Figure 11.1.1 11.1. 1: Periodic table by Dmitri Mendeleev, 1871. The valence electrons are the electrons that take part in the formation of a covalent bond with other atoms. The valence electrons are the electrons present in the outermost shell of an atom. 2) Now let us see how we can find out the valence electrons present in the nickel atom. The nickel is a transition element that is present in the .
Nickel-58 is the most abundant isotope of nickel, making up 68.077% of the natural abundance. Nickel-60 is composed of 28 protons, 32 neutrons, and 28 electrons. Nickel-61 is composed of 28 protons, 33 neutrons, and 28 electrons. Nickel-61 is the only stable isotope of nickel with a nuclear spin (I = 3/2), which makes it useful for studies by .
To write the orbital diagram of nickel, you have to write the orbital notation of nickel. Which has been discussed in detail above. Nickel orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electrons will first enter the 1s orbital.
nickel valence electrons|valence electrons chart
PH0 · valence electrons worksheet
PH1 · valence electrons chart
PH2 · periodic table valence electrons
PH3 · orbital diagram of nickel
PH4 · list of valence electrons for each element
PH5 · how to calculate valence electrons
PH6 · how many electrons in nickel
PH7 · full electron configuration of nickel
PH8 · Iba pa